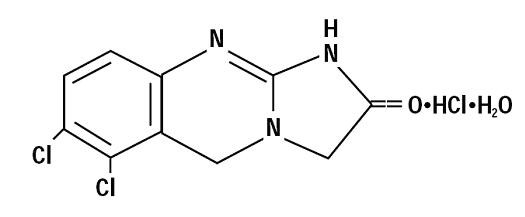
These highlights do not include all the information needed to use ANAGRELIDE CAPSULES safely and effectively. See full prescribing information for ANAGRELIDE CAPSULES. ANAGRELIDE capsules, for oral useInitial U.S. Approval: 1997

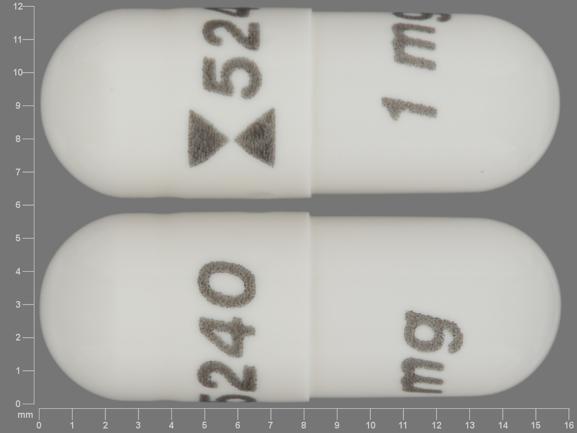
Teva Issues Voluntary Nationwide Recall of One Lot of Anagrelide Capsules, USP 0.5 mg Due to Dissolution Test Failure | FDA

FDA announces recall of platelet-reducing medication due to risk of clotting or other adverse cardiovascular outcomes

FDA announces recall of platelet-reducing medication due to risk of clotting or other adverse cardiovascular outcomes

Teva Recalls One Lot of Anagrelide Capsules, USP 0.5 mg Due to Dissolution Test Failure | American Pharmaceutical Review - The Review of American Pharmaceutical Business & Technology

These highlights do not include all the information needed to use ANAGRELIDE CAPSULES safely and effectively. See full prescribing information for ANAGRELIDE CAPSULES. ANAGRELIDE capsules, for oral useInitial U.S. Approval: 1997

Torrent Pharmaceuticals Recall Anagrelide Capsules over drug's Dissolution test Failure • Drugwatcher.org