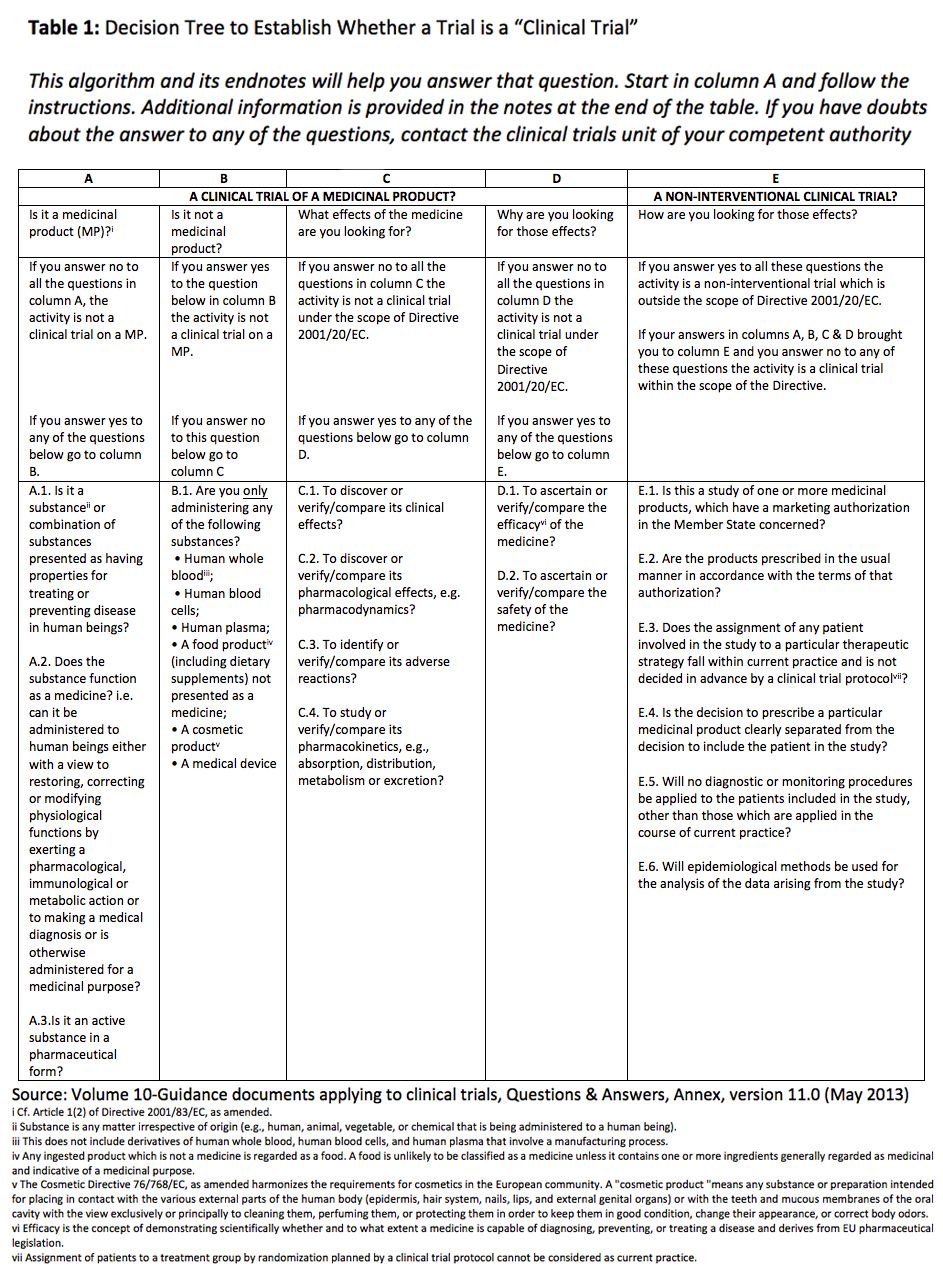
Guidance Notes for Applicants of the Certificate for Clinical Trial on Medical Device - PDF Free Download

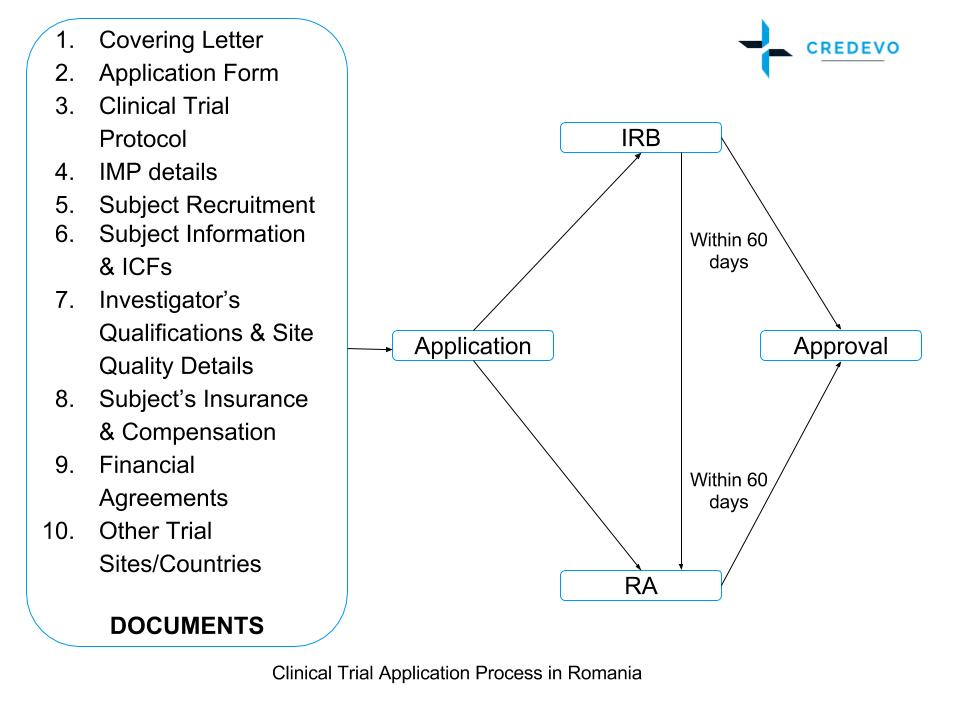
Guidance Document: Part C, Division 5 of the Food and Drug Regulations “Drugs for Clinical Trials Involving Human Subjects” (GUI-0100) - Canada.ca
CLINICAL TRIAL APPLICATION FORM African Vaccine Regulatory Forum (AVAREF) Clinical trial application form Trial's full title Sho
REQUEST FOR AUTHORISATION TO THE COMPETENT AUTHORITY: REQUEST FOR OPINION OF THE ETHICS COMMITTEE: A. TRIAL IDENTIFICATION
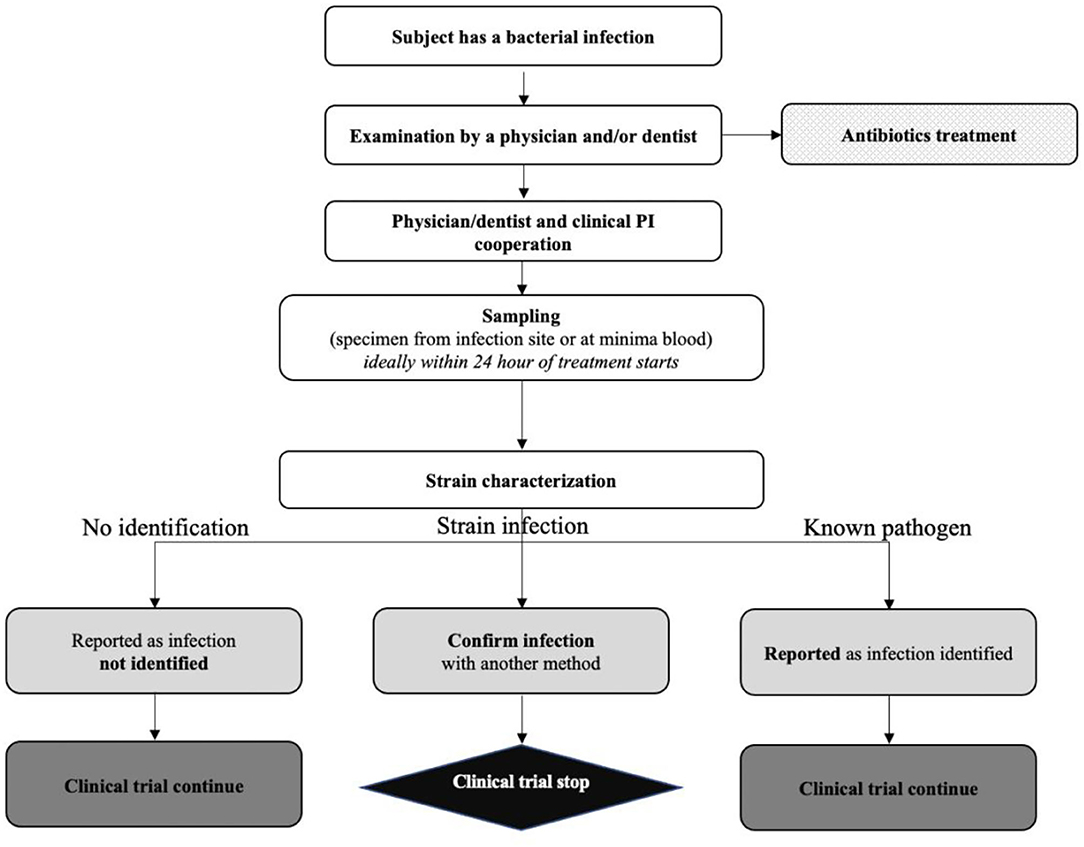
Frontiers | Entering First-in-Human Clinical Study With a Single-Strain Live Biotherapeutic Product: Input and Feedback Gained From the EMA and the FDA
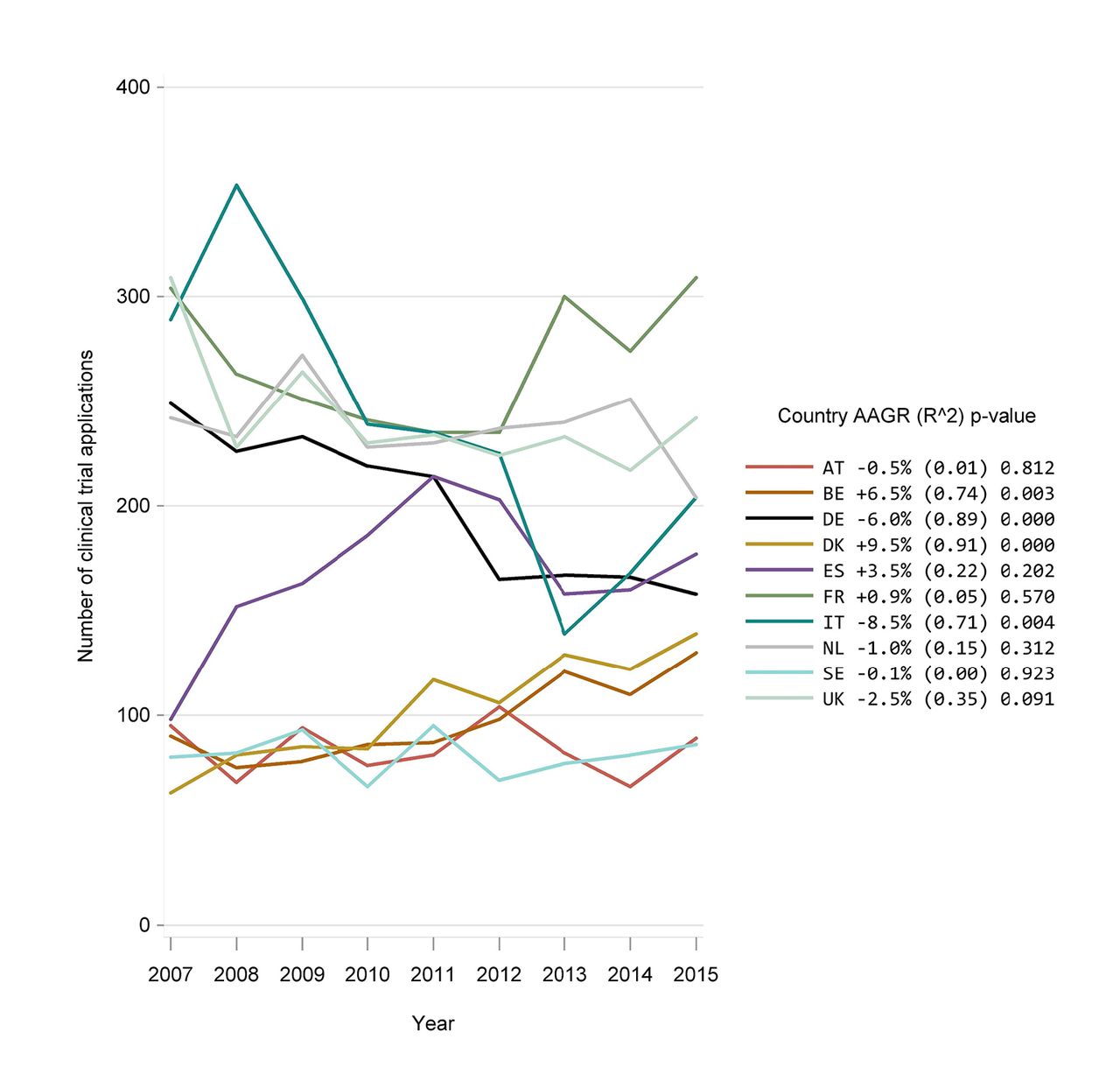
Development in the number of clinical trial applications in Western Europe from 2007 to 2015: retrospective study of data from national competent authorities | BMJ Open
REQUEST FOR AUTHORISATION TO THE COMPETENT AUTHORITY: REQUEST FOR OPINION OF THE ETHICS COMMITTEE: A. TRIAL IDENTIFICATION