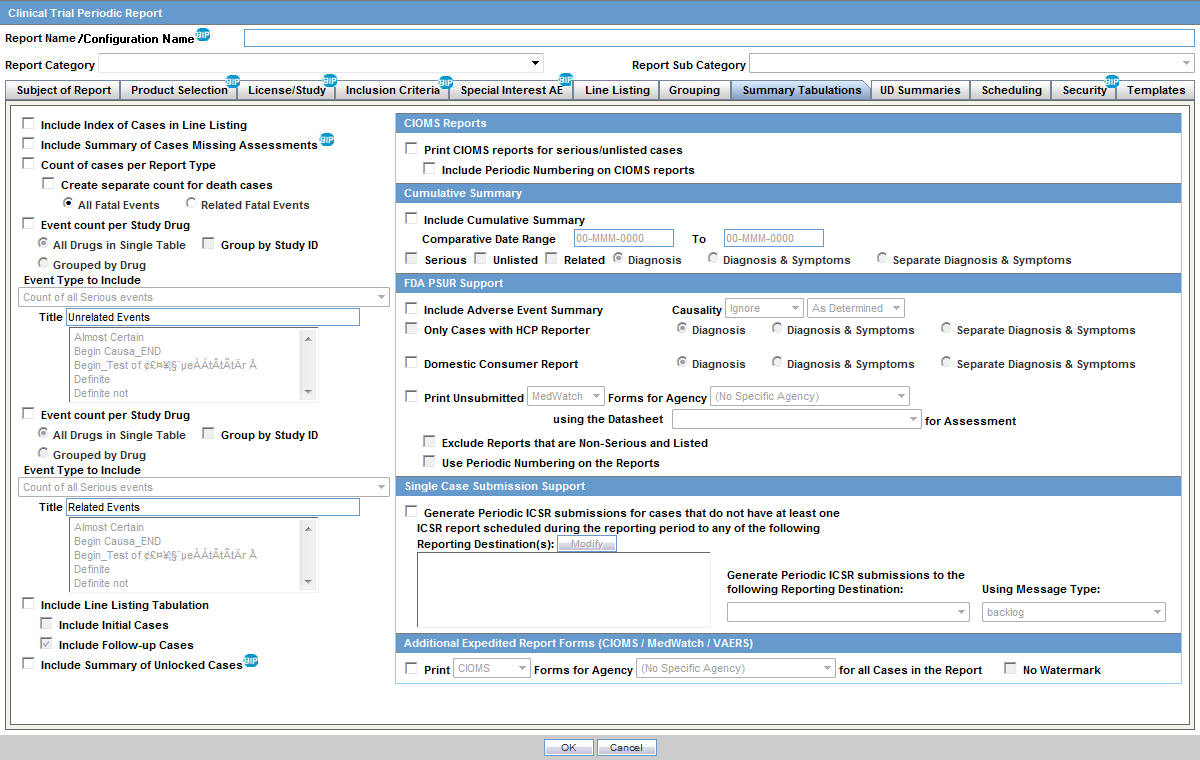
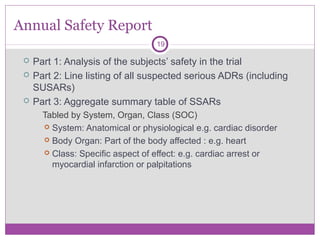
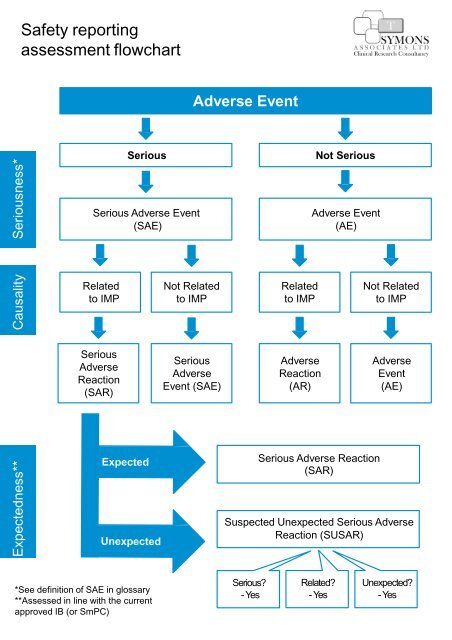
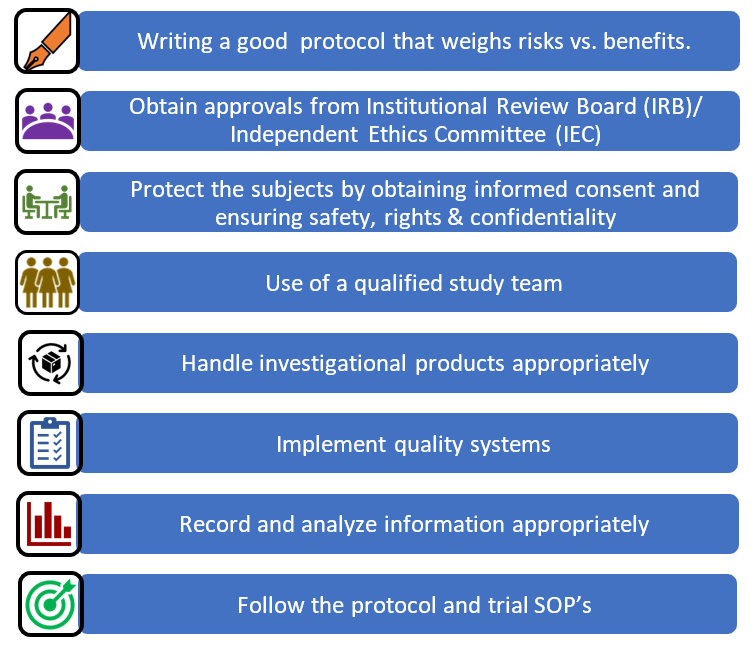
Safety Reporting Overload in Clinical Trials: FDA and Site Perspectives on Overreporting of Adverse Events | CenterWatch

European Medicines Agency on LinkedIn: Clinical Trials Information System: training and support - European…

Training log for new clinical research associates (CRAs). P&P, policies... | Download Scientific Diagram
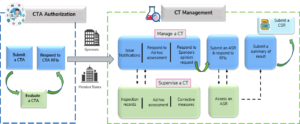
Adapting to the Evolving European Clinical Trial Regulatory Scenario: An Overview of the Current State of the European Clinical Trials Regulation and Clinical Trials Information System - ACRP