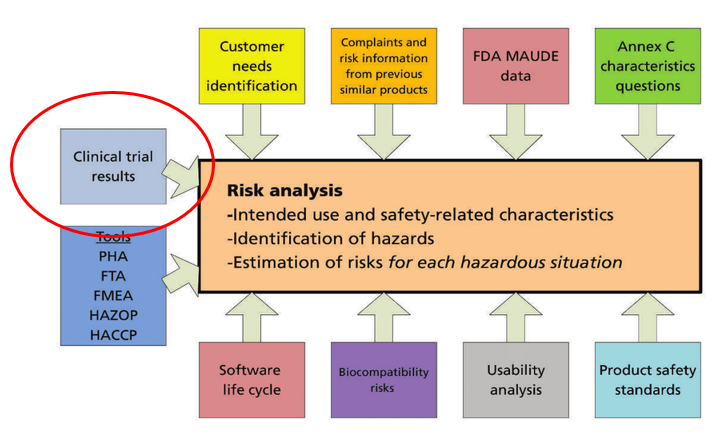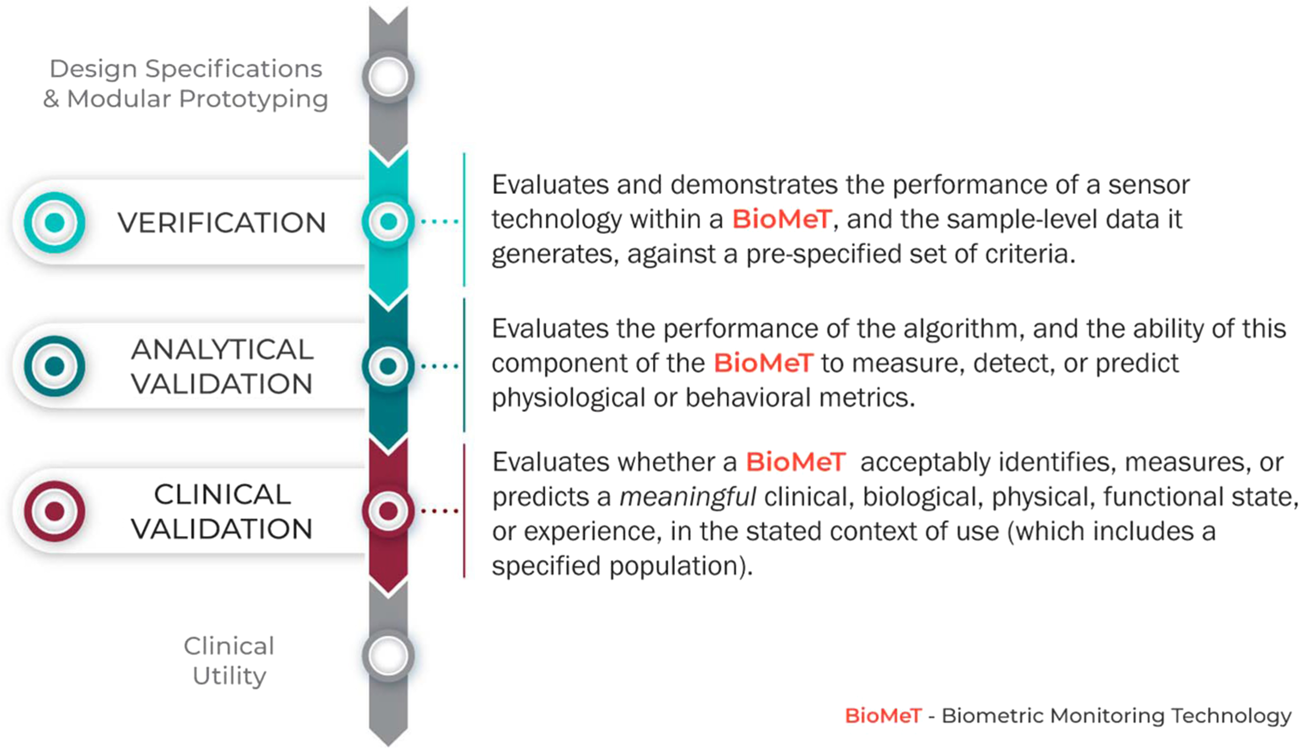
Verification, analytical validation, and clinical validation (V3): the foundation of determining fit-for-purpose for Biometric Monitoring Technologies (BioMeTs) | npj Digital Medicine
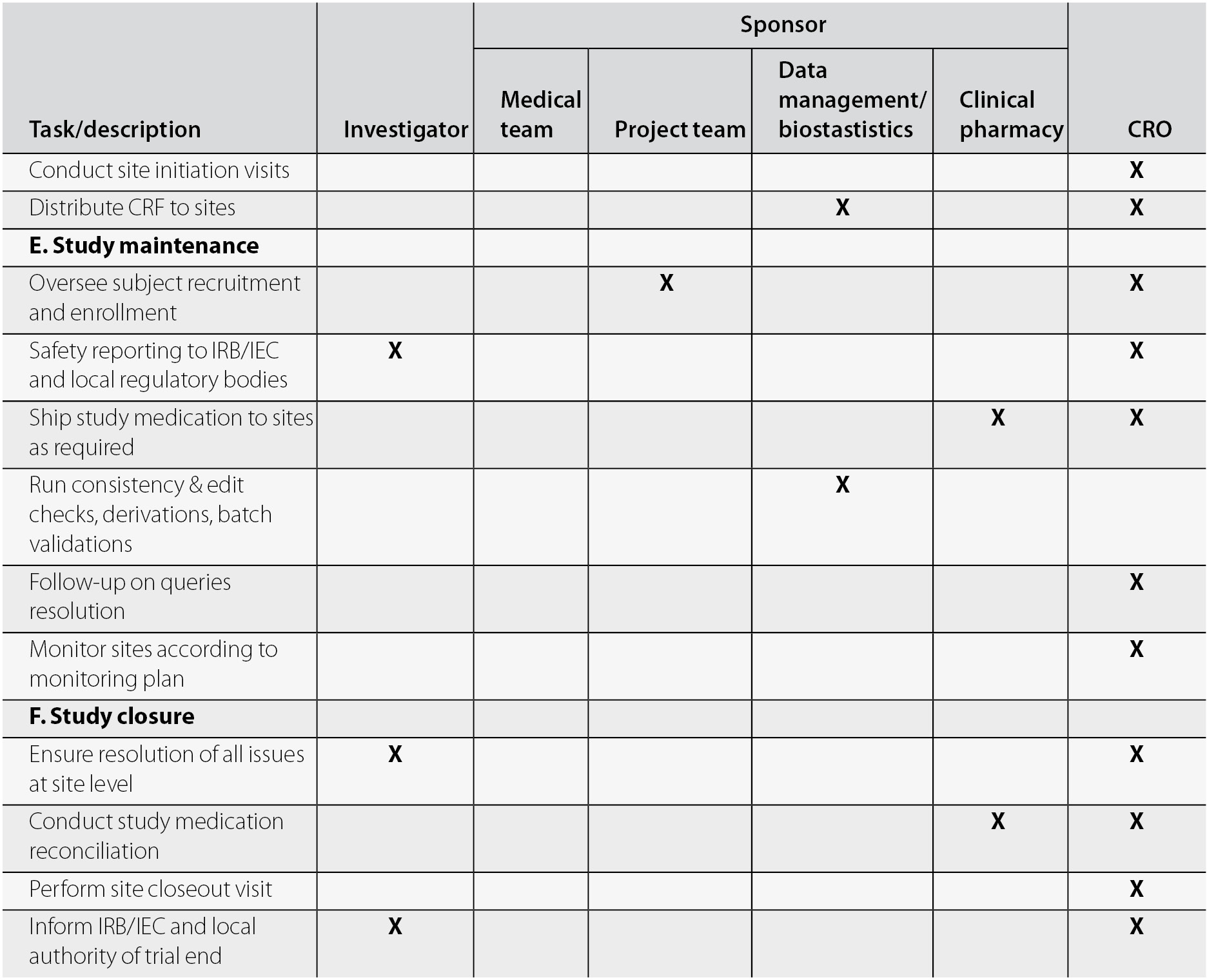
ich gcp 3.1.6 — Clinical Research Blog | Certified Clinical Research Professionals Society - Clinical Research Certification

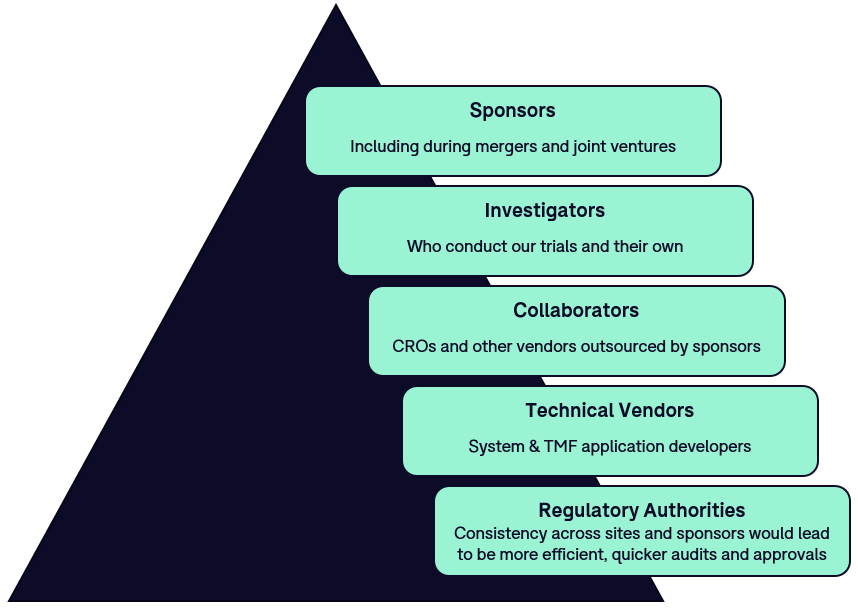
PDF) Central Institutional Ethics Committee needed to facilitate timely review of multicenter clinical trials

PDF) Central Institutional Ethics Committee needed to facilitate timely review of multicenter clinical trials
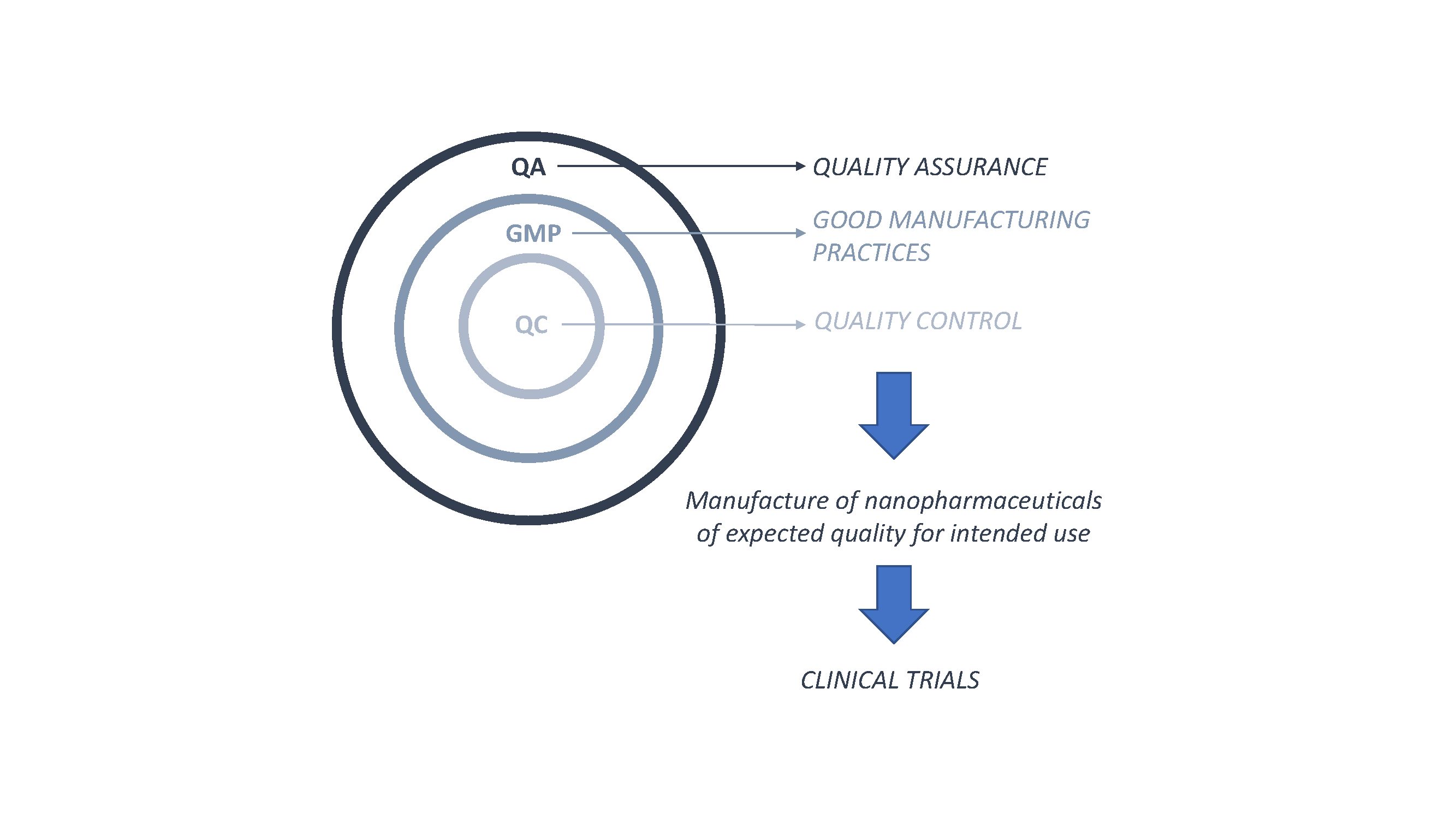
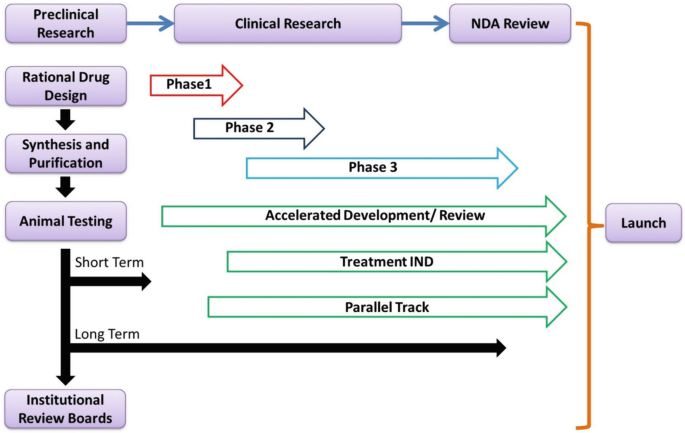
Pharmaceutics | Free Full-Text | Nanopharmaceutics: Part I—Clinical Trials Legislation and Good Manufacturing Practices (GMP) of Nanotherapeutics in the EU | HTML